Skip to written content Ecommerce Internet site is Are living now.. You should purchase any documents direct from our Store for the menu
These actions support be certain that the water system continues to operate in just specified parameters and fulfills the required excellent requirements.
A great way of accomplishing document inspections is to have a document schedule clearly indicating which files needs to be finished by when while in the challenge. Once the IQ is completed and reviewed, The end result is offered within the IQ report and, if no crucial deviations had been recognized, the OQ can start out.
July 2020 update: The guideline is up-to-date to reflect adjustments in the European Pharmacopoeia such as the revised monograph for Water for Injections permitting strategies aside from distillation for manufacturing water of injectable good quality.
Your browser isn’t supported anymore. Update it to obtain the most effective YouTube experience and our most current capabilities. Find out more
Thought paper on the need for revision of Take note for steering on high-quality of water for pharmaceutical use (H+V)
Of course. A temperature of 80˚C is very “forgiving” of cooler destinations which might nonetheless be sanitized In spite of a 10-15˚C temperature decline mainly because it penetrates all over the system by convection and conduction, so it is very efficient. Cooler temperatures (right down to sixty five˚C) may also be made use of but is “unforgiving” of nevertheless cooler places for instance outlet valves off of the principle loop. So these kinds of cooler destinations have to be flushed with this a little cooler sizzling water in an effort to assure that each one surfaces get to sanitizing temperatures larger than 60˚C.
Register to receive a daily e-mail of present day major military information tales from Stars and Stripes and leading news outlets from worldwide.
It is additionally important to steer clear of environment needs unnecessarily higher during get started-up, screening or operation that, on nearer inspection, usually do not need to be met. In rapidly-monitor assignments where time is a crucial variable, alterations and updates just take time and it is actually preferable to assess the set up cautiously At first click here in the necessities specification. A danger analysis regarding the end item (e.g., water quality) need to be carried out in advance of compiling the URS. The necessities referring to the protection of plant operators needs to be part of the risk analysis that occurs for CE marking with the installation, in accordance with the machinery directive.
If required a PDF Model also provided on the Engineering Office for required motion with the acquisition department and seller.
IQ is carried out to make certain the premises supporting utilities and products are already built and mounted in compliance with their permitted style and design specification (DQ) plus the maker’s guide and proposals.
There may be a likelihood of employing facts from close by water therapy systems for comparative reasons if the exact same feed water is for use.
ISPE customers situated in nations with emerging economies* are eligible to get a click here 50% discounted on publications through the standard member cost. To get the discounted, customers should be logged in with their ISPE member account.
Along with these Most important strategies, other purification steps for example deionization, carbon filtration, and UV disinfection tend to be included in WFI systems to be certain the highest excellent of water.
 Kel Mitchell Then & Now!
Kel Mitchell Then & Now!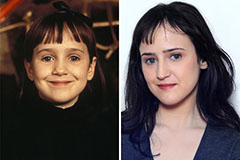 Mara Wilson Then & Now!
Mara Wilson Then & Now! Spencer Elden Then & Now!
Spencer Elden Then & Now! Sam Woods Then & Now!
Sam Woods Then & Now! Matilda Ledger Then & Now!
Matilda Ledger Then & Now!